Genomics England
Powered by SNPnexus
Contact our nurses
| Gene | Protein Alteration | Alteration Match | Drug | Effect | Tumour | Evidence |
|---|---|---|---|---|---|---|
| TMPRSS2 | K379R | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | K379R | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| FGFR2 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| AKT3 | intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| PDGFB | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | DFS | FDA guidelines |
| TP53 | R175H | Complete Match | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R175H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R175H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R175H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R175H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R175H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R175H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R175H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R175H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R175H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R175H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R175H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_varian | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_varian | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_varian | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| PDGFRA | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| JAK2 | intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| ESR1 | intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| MET | intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| BCR | intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ESR1 | 3-UTRSNV | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| EGFR | intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| MET | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| NRG1 | intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| TMPRSS2 | intron_variant | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| ERBB4 | intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| RET | intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| JAK2 | intron_variant), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| JAK2 | L712V), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_varian | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| STAG2 | E756* | Complete Match | PARP inhibitor | Responsive | G | Pre-clinical |
| BCR | 3-UTRSNV | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | 3-UTRSNV | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | 3-UTRSNV | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | 3-UTRSNV | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | 3-UTRSNV | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | 3-UTRSNV | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| TP53 | Y327* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y327* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y327* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y327* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y327* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y327* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y327* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y327* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y327* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y327* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y327* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y327* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NTRK3 | intron_variant), NTRK3 MUT* (D193V), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlockSubstitut | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (D193V), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlockSubstitut | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (D193V), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlockSubstitut | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | Pan-RAF inhibitor | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | U0126 (MEK inhibitor) | Responsive | PRAD | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| EGFR | intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| BRAF | E275Q | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | E275Q | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | E275Q | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | E275Q | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| PML | intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| TP53 | R248W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BRD4 | intron_variant | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| ALK | intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| NOTCH2 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| ABL1 | intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| PDGFRA | 3-UTRSNV | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| TP53 | E198* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E198* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E198* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E198* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E198* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E198* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E198* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E198* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E198* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E198* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E198* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E198* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E198* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E198* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E198* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| KMT2A | R160L | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | R160L | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | R160L | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | R160L | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | R160L | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | R160L | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| NOTCH1 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | 3-UTRSNV | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| MET | intron_variant), MET MUT* (IntronicBlockSubstitution | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NOTCH2 | intron_variant), NOTCH2 MUT* (Q1136E), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant), N | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| TP53 | P278L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P278L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P278L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P278L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P278L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P278L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P278L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P278L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P278L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| PTEN | R130P | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130P | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130P | Different Mutation | BYL719 (PIK3CA inhibitor) | Resistant | BRCA | Case report |
| PTEN | R130P | Complete Match | ATM inhibitor | Responsive | BRCA | Pre-clinical |
| PTEN | R130P | Complete Match | PI3K pathway inhibitor | Responsive | BRCA, ED, TH, CANCER, L, G, OV | Pre-clinical |
| PTEN | R130P | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130P | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130P | Complete Match | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130P | Complete Match | PD1 Ab inhibitor | Resistant | CM | Early trials |
| PTEN | R130P | Complete Match | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130P | Complete Match | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130P | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | R130P | Complete Match | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130P | Complete Match | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130P | Complete Match | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130P | Different Alteration | PI3K pathway inhibitor | Responsive | L, BRCA, CANCER, G, ED, TH, OV | Pre-clinical |
| PTEN | R130P | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130P | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130P | Different Alteration | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130P | Different Alteration | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130P | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130P | Different Alteration | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130P | Different Alteration | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130P | Different Alteration | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130P | Different Alteration | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | IntronicBlockSubstitution), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_v | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| TP53 | D281N | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | D281N | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | D281N | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | D281N | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | D281N | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | D281N | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D281N | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | D281N | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | D281N | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281N | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | D281N | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281N | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), AL | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| MET | IntronicBlockSubstitution), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| KMT2A | intron_variant | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | intron_variant | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | intron_variant | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | intron_variant | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | intron_variant | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| FGFR3 | S344F | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | S344F | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | S344F | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| TP53 | R273H | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| JAK2 | intron_variant), JAK2 MUT* (5-UTRSNV | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| EGFR | M1007I | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | M1007I | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| NTRK3 | intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| BRCA2 | L2480* | Complete Match | PD1 Ab inhibitor | Responsive | CM | Case report |
| BRCA2 | L2480* | Complete Match | Olaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | L2480* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | OV | Late trials |
| BRCA2 | L2480* | Complete Match | Chemotherapy (PARP inhibitor;Chemotherapy) | Responsive | OV | Early trials |
| BRCA2 | L2480* | Complete Match | PARP inhibitor;Chemotherapy | Responsive | OV | Early trials |
| BRCA2 | L2480* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | PA | Case report |
| BRCA2 | L2480* | Complete Match | Olaparib (PARP inhibitor) | Responsive | PRAD | Early trials |
| BRCA2 | L2480* | Complete Match | Rucaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | L2480* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | L2480* | Complete Match | Veliparib;Cisplatin (PARP inhibitor;Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | L2480* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | L2480* | Complete Match | PARP inhibitor | Responsive | BRCA | Early trials |
| BRCA2 | L2480* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | L2480* | Different Alteration | PARP inhibitor | Responsive | OV | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (3-UTRSNV | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| CRLF2 | intron_variant | Different Alteration | MTOR inhibitor | Responsive | ALL | Pre-clinical |
| CRLF2 | intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH2 | R2298R | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| TP53 | L257P | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | L257P | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | L257P | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | L257P | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | L257P | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | L257P | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L257P | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | L257P | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | L257P | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L257P | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | L257P | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L257P | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (IntronicBlockSubstitution | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| TP53 | splice_donor_variant | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | splice_donor_variant | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | splice_donor_variant | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | splice_donor_variant | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | splice_donor_variant | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_donor_variant | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | splice_donor_variant | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | splice_donor_variant | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| PTEN | splice_acceptor_variant | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | splice_acceptor_variant | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | splice_acceptor_variant | Different Mutation | BYL719 (PIK3CA inhibitor) | Resistant | BRCA | Case report |
| PTEN | splice_acceptor_variant | Complete Match | ATM inhibitor | Responsive | BRCA | Pre-clinical |
| PTEN | splice_acceptor_variant | Complete Match | PI3K pathway inhibitor | Responsive | BRCA, ED, TH, CANCER, L, G, OV | Pre-clinical |
| PTEN | splice_acceptor_variant | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | splice_acceptor_variant | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | splice_acceptor_variant | Complete Match | PARP inhibitor | Responsive | ED | Case report |
| PTEN | splice_acceptor_variant | Complete Match | PD1 Ab inhibitor | Resistant | CM | Early trials |
| PTEN | splice_acceptor_variant | Complete Match | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | splice_acceptor_variant | Complete Match | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | splice_acceptor_variant | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | splice_acceptor_variant | Complete Match | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | splice_acceptor_variant | Complete Match | AKT inhibitor | Responsive | PA | Case report |
| PTEN | splice_acceptor_variant | Complete Match | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | splice_acceptor_variant | Different Alteration | PI3K pathway inhibitor | Responsive | L, BRCA, CANCER, G, ED, TH, OV | Pre-clinical |
| PTEN | splice_acceptor_variant | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | splice_acceptor_variant | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | splice_acceptor_variant | Different Alteration | PARP inhibitor | Responsive | ED | Case report |
| PTEN | splice_acceptor_variant | Different Alteration | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | splice_acceptor_variant | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | splice_acceptor_variant | Different Alteration | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | splice_acceptor_variant | Different Alteration | AKT inhibitor | Responsive | PA | Case report |
| PTEN | splice_acceptor_variant | Different Alteration | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | splice_acceptor_variant | Different Alteration | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor | Responsive | CANCER | Early trials |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor;CDK4 inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor;MTOR inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor | Responsive;Responsive | ALL | Early trials;Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Complete Match | NOTCH1 inhibitor | Responsive | ADCC | Case report |
| NOTCH1 | H2428Pfs*5 | Complete Match | OMP-52M51 (NOTCH1 inhibitor) | Responsive | CANCER | Early trials |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Complete Match | Gamma secretase inhibitor | Responsive | MCL | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | H2428Pfs*5 | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| TP53 | I255T | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | I255T | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | I255T | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | I255T | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | I255T | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | I255T | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | I255T | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | I255T | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | I255T | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | I255T | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | I255T | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | I255T | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | I255T | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | I255T | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | I255T | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| FGFR3 | 3-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | 3-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | 3-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| PTEN | R130Q | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130Q | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130Q | Different Mutation | BYL719 (PIK3CA inhibitor) | Resistant | BRCA | Case report |
| PTEN | R130Q | Complete Match | ATM inhibitor | Responsive | BRCA | Pre-clinical |
| PTEN | R130Q | Complete Match | PI3K pathway inhibitor | Responsive | BRCA, ED, TH, CANCER, L, G, OV | Pre-clinical |
| PTEN | R130Q | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130Q | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130Q | Complete Match | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130Q | Complete Match | PD1 Ab inhibitor | Resistant | CM | Early trials |
| PTEN | R130Q | Complete Match | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130Q | Complete Match | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130Q | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | R130Q | Complete Match | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130Q | Complete Match | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130Q | Complete Match | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130Q | Different Alteration | PI3K pathway inhibitor | Responsive | L, BRCA, CANCER, G, ED, TH, OV | Pre-clinical |
| PTEN | R130Q | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130Q | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130Q | Different Alteration | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130Q | Different Alteration | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130Q | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130Q | Different Alteration | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130Q | Different Alteration | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130Q | Different Alteration | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130Q | Different Alteration | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| MET | IntronicBlockSubstitution | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| JAK2 | IntronicBlockSubstitution | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| NOTCH1 | Q1766H | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | Q1766H | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| FGFR2 | 5-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | 5-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| KMT2D | R1252* | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| EGFR | 3-UTRSNV | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | 3-UTRSNV | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| TP53 | P278S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P278S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P278S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P278S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P278S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P278S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P278S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P278S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P278S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ESR1 | 3-UTRSNV), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| TP53 | V216M | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V216M | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V216M | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V216M | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V216M | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V216M | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V216M | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V216M | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V216M | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V216M | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V216M | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V216M | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V216M | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V216M | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V216M | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BRAF | intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| PIK3CA | H1047R | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | H1047R | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | H1047R | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | H1047R | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | H1047R | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | H1047R | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | H1047R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | H1047R | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| EGFR | S229C | Different Alteration | Cetuximab (EGFR mAb inhibitor) | Responsive | COREAD | FDA guidelines |
| EGFR | S229C | Different Alteration | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| EGFR | S229C | Different Mutation | Rindopepimut (Vaccine) | Responsive | GB | Late trials |
| EGFR | S229C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | S229C | Different Mutation | Gefitinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | S229C | Different Mutation | HSP90 inhibitor | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Afatinib;Cetuximab (ERBB2 & EGFR inhibitor;EGFR mAb inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | MEK inhibitor | Responsive | L | Pre-clinical |
| EGFR | S229C | Different Mutation | EGFR TK inhibitor | Responsive | L | Late trials |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | NCCN guidelines |
| EGFR | S229C | Different Mutation | Osimertinib (EGFR inhibitor) | Resistant | L | Early trials |
| EGFR | S229C | Different Mutation | EGFR-TKI's (EGFR inhibitor) | Resistant | L | Late trials |
| EGFR | S229C | Different Mutation | HSP90 inhibitor | Responsive | L | Case report |
| EGFR | S229C | Different Mutation | EGFR inhibitor | Resistant | L | Late trials |
| EGFR | S229C | Different Mutation | Poziotinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | L | Pre-clinical |
| EGFR | S229C | Different Mutation | HSP90 inhibitor (HSP90 inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | G | Pre-clinical |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | S229C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Rociletinib (EGFR inhibitor) | Resistant | LUAD | Case report |
| EGFR | S229C | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Responsive | COREAD | Case report |
| EGFR | S229C | Different Mutation | novel EGFR mAb inhibitor | Responsive | COREAD | Early trials |
| EGFR | S229C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| EGFR | S229C | Different Mutation | Cetuximab;Sirolimus (EGFR mAb inhibitor;MTOR inhibitor) | Responsive | HNC | Case report |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | S229C | Different Mutation | Gefitinib (EGFR inhibitor) | No Responsive | HNC | Case report |
| EGFR | S229C | Different Mutation | EGFR inhibitor | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Resistant | L | NCCN/CAP guidelines |
| EGFR | S229C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | S229C | Different Mutation | EGFR inhibitor | Responsive | NSCLC | Late trials |
| EGFR | S229C | Different Mutation | Afatinib;Nimotuzumab (ERBB2 & EGFR inhibitor;EGFR mAb inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Resistant | L | NCCN/CAP guidelines |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | S229C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | L | Late trials |
| EGFR | S229C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Pre-clinical |
| EGFR | S229C | Different Mutation | Erlotinib (EGFR inhibitor) | No Responsive | L | Case report |
| EGFR | S229C | Different Mutation | Lapatinib (ERBB2 inhibitor) | Responsive | ED | Case report |
| EGFR | S229C | Different Mutation | EGFR inhibitor | No Responsive | G | Early trials |
| EGFR | S229C | Different Mutation | EGFR inhibitor | Resistant | NSCLC | Case report |
| EGFR | S229C | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| EGFR | S229C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant;Resistant | COREAD | Pre-clinical;Case report |
| EGFR | S229C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Responsive | HNC | Case report |
| EGFR | S229C | Different Alteration | Gefitinib (EGFR inhibitor) | Responsive | ED | Late trials |
| EGFR | S229C | Different Alteration | Gefitinib (EGFR inhibitor) | No Responsive | HNC | Early trials |
| EGFR | S229C | Different Alteration | EGFR inhibitor | Responsive | HNSC | Case report |
| EGFR | S229C | Different Alteration | EGFR inhibitor | No Responsive | G | Early trials |
| EGFR | S229C | Different Alteration | Erlotinib (EGFR inhibitor) | No Responsive | L | Early trials |
| EGFR | S229C | Different Alteration | EGFR mAb inhibitor | Responsive | COREAD | Late trials |
| EGFR | S229C | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | S229C | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Alteration | Pertuzumab (ERBB2 mAb inhibitor) | Responsive | BRCA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | BRCA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | ST, BRCA, GEJA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | Ado-Trastuzumab Emtansine (ERBB2 mAb inhibitor) | Responsive | BRCA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | MK2206;Trastuzumab (allosteric AKT inhibitor;ERBB2 mAb inhibitor) | Responsive | SOLID | Early trials |
| ERBB2 | V777L | Complete Match | Lapatinib (ERBB2 inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Complete Match | Neratinib (ERBB2 inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Mutation | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | CANCER, BRCA | Pre-clinical |
| ERBB2 | V777L | Different Mutation | Neratinib (ERBB2 inhibitor) | Responsive | CANCER | Early trials |
| ERBB2 | V777L | Complete Match | Neratinib (ERBB2 inhibitor) | Responsive | CANCER | Early trials |
| ERBB2 | V777L | Different Mutation | Neratinib (ERBB2 inhibitor) | Responsive | BRCA | Case report |
| ERBB2 | V777L | Different Mutation | Lapatinib (ERBB2 inhibitor) | Responsive | BRCA, LUAD | Case report |
| ERBB2 | V777L | Different Mutation | Ado-Trastuzumab Emtansine (ERBB2 mAb inhibitor) | Responsive | L | Early trials |
| ERBB2 | V777L | Different Mutation | Neratinib (ERBB2 inhibitor) | Resistant | BRCA | Case report |
| ERBB2 | V777L | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Mutation | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | LUAD | Case report |
| ERBB2 | V777L | Different Mutation | Neratinib (ERBB2 inhibitor) | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Mutation | Lapatinib (ERBB2 inhibitor) | Resistant | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Mutation | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | CANCER | Pre-clinical |
| ERBB2 | V777L | Complete Match | Temsirolimus (MTOR inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Complete Match | Dacomitinib (Pan ERBB inhibitor) | Responsive | NSCLC | Early trials |
| ERBB2 | V777L | Complete Match | Neratinib (ERBB2 inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | ERBB2 inhibitor | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | ERBB2 mAb inhibitor | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | Neratinib (ERBB2 inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Mutation | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | LUAD | Early trials |
| ERBB2 | V777L | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | BRCA | Late trials |
| ERBB2 | V777L | Different Alteration | Pertuzumab (ERBB2 mAb inhibitor) | Responsive | BT | Early trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Responsive | COREAD | Late trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | OV | Early trials |
| ERBB2 | V777L | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | BLCA | Case report |
| ERBB2 | V777L | Different Alteration | Ado-Trastuzumab Emtansine (ERBB2 mAb inhibitor) | Responsive | COREAD | Case report |
| ERBB2 | V777L | Different Alteration | Rociletinib (EGFR inhibitor) | Resistant | LUAD | Case report |
| ERBB2 | V777L | Different Alteration | Osimertinib (EGFR inhibitor) | Resistant | LUAD | Case report |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | BT | Early trials |
| ERBB2 | V777L | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Early trials |
| ERBB2 | V777L | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | BT | Early trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab;HSP90 inhibitor (ERBB2 mAb inhibitor;HSP90 inhibitor) | Responsive | BRCA | Early trials |
| ERBB2 | V777L | Different Alteration | HER2 inhibitor;CDK4/6 inhibitor | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | BRCA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | Pertuzumab (ERBB2 mAb inhibitor) | Responsive | ST | Early trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab;Everolimus;Chemotherapy (ERBB2 mAb inhibitor;MTOR inhibitor;Chemotherapy) | Responsive | BRCA | Early trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | ST, GEJA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | ERBB2 inhibitor;CDK4/6 inhibitor | Responsive | BRCA | Pre-clinical |
| ERBB2 | V777L | Different Alteration | Ado-Trastuzumab Emtansine (EGFR mAb inhibitor) | Responsive | BRCA | FDA guidelines |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | Responsive | HNC | Case report |
| ERBB2 | V777L | Different Alteration | Neratinib (ERBB2 inhibitor) | Responsive | BRCA | Late trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab (ERBB2 mAb inhibitor) | No Responsive | ED | Early trials |
| ERBB2 | V777L | Different Alteration | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Responsive | ED | Pre-clinical |
| ERBB2 | V777L | Different Alteration | Lapatinib;Chemotherapy (ERBB2 inhibitor;Chemotherapy) | Responsive | ST | Early trials |
| ERBB2 | V777L | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | ST | Case report |
| ERBB2 | V777L | Different Alteration | EGFR inhibitor | Resistant | LUAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| ERBB4 | IntronicBlockSubstitution), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| TP53 | R248G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| HRAS | Q61H | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | CER | Pre-clinical |
| HRAS | Q61H | Complete Match | MEK inhibitor +/- MTOR inhibitor | Responsive | AML | Pre-clinical |
| HRAS | Q61H | Complete Match | MTOR inhibitor | Responsive | CESC | Pre-clinical |
| HRAS | Q61H | Complete Match | Tipifarnib (Farnesyltransferase inhibitor) | Responsive | CANCER | Early trials |
| PML | IntronicBlockSubstitution | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | IntronicBlockSubstitution | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | IntronicBlockSubstitution | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| JAK2 | 3-UTRSNV | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| JAK2 | P98R | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| TP53 | Q104* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q104* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q104* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q104* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q104* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q104* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q104* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q104* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q104* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q104* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q104* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q104* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NTRK1 | IntronicBlockSubstitution | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | IntronicBlockSubstitution | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | IntronicBlockSubstitution | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | IntronicBlockSubstitution | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| TP53 | R158P | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R158P | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R158P | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R158P | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R158P | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R158P | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R158P | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R158P | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R158P | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158P | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R158P | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158P | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| LRP1B | splice_acceptor_variant | Complete Match | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | splice_acceptor_variant | Different Alteration | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlock | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlock | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (IntronicBlock | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| PDGFRA | IntronicBlockSubstitution), PDGFRA MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248Q), TP53 MUT (R248Q | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q), TP53 MUT (R248Q | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BCR | IntronicBlockSubstitution | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | IntronicBlockSubstitution | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| TP53 | R282W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R282W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R282W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R282W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R282W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R282W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R282W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R282W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R282W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R282W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| TP53 | V274F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V274F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V274F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V274F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V274F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V274F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V274F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V274F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V274F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V274F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V274F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V274F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V274F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V274F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V274F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| KMT2D | Q3682* | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| SMARCA4 | splice_donor_variant | Complete Match | EZH2 inhibitor | Responsive | OVRT | Case report |
| SMARCA4 | splice_donor_variant | Complete Match | AURKA inhibitor | Responsive | NSCLC | Pre-clinical |
| SMARCA4 | splice_donor_variant | Complete Match | EZH2 inhibitor | Responsive | OV | Case report |
| SMARCA4 | splice_donor_variant | Different Alteration | EZH2 inhibitor | Responsive | OVRT | Case report |
| SMARCA4 | splice_donor_variant | Different Alteration | EZH2 inhibitor | Responsive | OV | Case report |
| PML | 3-UTRSNV | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | 3-UTRSNV | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | 3-UTRSNV | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| TP53 | E294* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E294* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E294* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E294* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E294* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E294* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E294* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E294* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E294* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E294* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E294* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E294* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BRD4 | E613K | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (N787H | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (3-UTRSNV | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| APC | L1601* | Complete Match | Tankyrase inhibitor | Responsive | COREAD | Pre-clinical |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| TP53 | L194R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | L194R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | L194R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | L194R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | L194R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | L194R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L194R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L194R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L194R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | L194R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | L194R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | L194R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L194R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | L194R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L194R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W53* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | W53* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | W53* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | W53* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | W53* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | W53* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W53* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | W53* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | W53* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W53* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | W53* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W53* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y220C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y220C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y220C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y220C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y220C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y220C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y220C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y220C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NF1 | splice_donor_variant | Different Mutation | Vemurafenib (BRAF inhibitor) | Resistant | CM | Pre-clinical |
| NF1 | splice_donor_variant | Different Mutation | Everolimus (MTOR inhibitor) | Responsive | HNC | Case report |
| NF1 | splice_donor_variant | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Complete Match | Vinblastine (Chemotherapy) | Responsive | G | Early trials |
| NF1 | splice_donor_variant | Complete Match | KIT inhibitor;MTOR inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Tamoxifen (Hormonal therapy) | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Bevacizumab (VEGFR mAb inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Complete Match | Retinoic Acid | Resistant | NB | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Everolimus (MTOR inhibitor) | Responsive | NF | Late trials |
| NF1 | splice_donor_variant | Complete Match | Trametinib (MEK inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Complete Match | Sorafenib;Sirolimus (Pan-TK inhibitor;MTOR inhibitor) | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Nilotinib (BCR-ABL inhibitor) | Responsive | MPN, PLEN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | MEK inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Everolimus (MTOR inhibitor) | Responsive | HNC, SG | Case report |
| NF1 | splice_donor_variant | Complete Match | Everolimus;Bevacizumab (MTOR inhibitor;VEGFR mAb inhibitor) | No Responsive | MPN | Early trials |
| NF1 | splice_donor_variant | Complete Match | PLX3397 (Pan-TK inhibitor) | Responsive | PLEN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | MEK inhibitor | Responsive | LK, G | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Pan-RAF inhibitor;MEK inhibitor | Responsive | CM | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Complete Match | AURK inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | MTOR inhibitor | Responsive | LK, G, MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Dasatinib (BCR-ABL inhibitor) | Resistant | L | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Everolimus;Pazopanib (MTOR inhibitor;VEGFR inhibitor) | Responsive | HC | Case report |
| NF1 | splice_donor_variant | Complete Match | Everolimus (MTOR inhibitor) | No Responsive | MPN | Early trials |
| NF1 | splice_donor_variant | Complete Match | Cediranib (ALK inhibitor) | No Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Complete Match | PD1 Ab inhibitor | Responsive | CM | Early trials |
| NF1 | splice_donor_variant | Complete Match | Vinblastine;Nilotinib (Chemotherapy;BCR-ABL inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Complete Match | Sirolimus;Erlotinib (MTOR inhibitor;EGFR inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Complete Match | Everolimus;Erlotinib (MTOR inhibitor;EGFR inhibitor) | No Responsive | G | Case report |
| NF1 | splice_donor_variant | Complete Match | BRD4 inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Tipifarnib (Farnesyltransferase inhibitor) | No Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Complete Match | Selumetinib (MEK inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Complete Match | MTOR inhibitor;HSP90 inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Trametinib (MEK inhibitor) | Responsive | CM | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Erlotinib (EGFR inhibitor) | Resistant | L | Pre-clinical |
| NF1 | splice_donor_variant | Complete Match | Trametinib (MEK inhibitor) | No Responsive | OS | Case report |
| NF1 | splice_donor_variant | Different Alteration | MTOR inhibitor | Responsive | MPN, G, LK | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | PLX3397 (Pan-TK inhibitor) | Responsive | PLEN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Resistant | L | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Trametinib (MEK inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Different Alteration | BRD4 inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Trametinib (MEK inhibitor) | No Responsive | OS | Case report |
| NF1 | splice_donor_variant | Different Alteration | Cediranib (ALK inhibitor) | No Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Bevacizumab (VEGFR mAb inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Different Alteration | MEK inhibitor | Responsive | G, LK | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Vinblastine (Chemotherapy) | Responsive | G | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Sorafenib;Sirolimus (Pan-TK inhibitor;MTOR inhibitor) | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Everolimus;Bevacizumab (MTOR inhibitor;VEGFR mAb inhibitor) | No Responsive | MPN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Resistant | L | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | MTOR inhibitor;HSP90 inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Sirolimus;Erlotinib (MTOR inhibitor;EGFR inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Tamoxifen (Hormonal therapy) | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | SG, HNC | Case report |
| NF1 | splice_donor_variant | Different Alteration | Pan-RAF inhibitor;MEK inhibitor | Responsive | CM | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Tipifarnib (Farnesyltransferase inhibitor) | No Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Retinoic Acid | Resistant | NB | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Everolimus (MTOR inhibitor) | No Responsive | MPN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | PLEN | Early trials |
| NF1 | splice_donor_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | PLEN, MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Vinblastine;Nilotinib (Chemotherapy;BCR-ABL inhibitor) | Responsive | G | Case report |
| NF1 | splice_donor_variant | Different Alteration | Trametinib (MEK inhibitor) | Responsive | CM | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | AURK inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | Everolimus;Erlotinib (MTOR inhibitor;EGFR inhibitor) | No Responsive | G | Case report |
| NF1 | splice_donor_variant | Different Alteration | PD1 Ab inhibitor | Responsive | CM | Early trials |
| NF1 | splice_donor_variant | Different Alteration | MEK inhibitor | Responsive | MPN | Pre-clinical |
| NF1 | splice_donor_variant | Different Alteration | KIT inhibitor;MTOR inhibitor | Responsive | MPN | Pre-clinical |
| PIK3CA | N345K | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | N345K | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | N345K | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | N345K | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | N345K | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | N345K | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | N345K | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | N345K | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| KRAS | G12A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12A | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12A | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12A | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12A | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12A | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12A | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12A | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12A | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12A | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12A | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12A | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12A | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12A | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12A | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12A | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| AKT3 | P206P | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| TP53 | R273G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NRG1 | H364H), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | H364H), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| CDH1 | Q23* | Complete Match | Bicalutamide (AR inhibitor) | Responsive | BRCA | Pre-clinical |
| NRG1 | 5-UTRSNV | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | 5-UTRSNV | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| TMPRSS2 | H77Y | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | H77Y | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| TP53 | R248Q | Complete Match | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248Q | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248Q | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248Q | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248Q | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248Q | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248Q | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248Q | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248Q | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BCR | E195D | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | E195D | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | E195D | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | E195D | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | E195D | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | E195D | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| RB1 | I246Hfs*12 | Different Alteration | Palbociclib (CDK4/6 inhibitor) | Responsive | PRAD | Pre-clinical |
| RB1 | I246Hfs*12 | Complete Match | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | I246Hfs*12 | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | I246Hfs*12 | Complete Match | HDAC inhibitor | Responsive | RB | Pre-clinical |
| RB1 | I246Hfs*12 | Different Alteration | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | I246Hfs*12 | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | I246Hfs*12 | Different Alteration | HDAC inhibitor | Responsive | RB | Pre-clinical |
| TP53 | M246R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | M246R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | M246R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | M246R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | M246R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | M246R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | M246R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M246R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M246R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | M246R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | M246R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | M246R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M246R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | M246R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M246R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| NTRK1 | G219G), NTRK1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | G219G), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | G219G), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | G219G), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| ESR1 | IntronicBlockSubstitution | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| TP53 | H193R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | H193R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | H193R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | H193R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | H193R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | H193R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H193R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H193R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H193R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | H193R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | H193R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | H193R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H193R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | H193R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H193R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BRD4 | Q266Q), BRD4 MUT* (IntronicBlockSubstitution), BRD4 MUT* (IntronicBlockSubstitution | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| TP53 | R306* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R306* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R306* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R306* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R306* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R306* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R306* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R306* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R306* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R306* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R306* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R306* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | 3-UTRSNV | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | 3-UTRSNV | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | 3-UTRSNV | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| MET | intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| TP53 | R280K | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R280K | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R280K | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R280K | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R280K | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R280K | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R280K | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R280K | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R280K | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R280K | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R280K | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R280K | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R280K | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R280K | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R280K | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C141Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C141Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C141Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C141Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C141Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C141Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C141Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C141Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C141Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C141Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C141Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C141Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (3-UTRSNV | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| FBXW7 | R505C | Complete Match | Steroid | Responsive | ALL | Late trials |
| FBXW7 | R505C | Complete Match | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | R505C | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | COREAD | Pre-clinical |
| FBXW7 | R505C | Different Alteration | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| MET | V1289M | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| FBXW7 | splice_donor_variant | Complete Match | Steroid | Responsive | ALL | Late trials |
| FBXW7 | splice_donor_variant | Complete Match | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | splice_donor_variant | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | COREAD | Pre-clinical |
| FBXW7 | splice_donor_variant | Different Alteration | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| TP53 | Y126C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y126C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y126C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y126C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y126C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y126C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y126C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y126C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y126C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y126C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y126C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y126C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BRCA2 | E731* | Complete Match | PD1 Ab inhibitor | Responsive | CM | Case report |
| BRCA2 | E731* | Complete Match | Olaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | E731* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | OV | Late trials |
| BRCA2 | E731* | Complete Match | Chemotherapy (PARP inhibitor;Chemotherapy) | Responsive | OV | Early trials |
| BRCA2 | E731* | Complete Match | PARP inhibitor;Chemotherapy | Responsive | OV | Early trials |
| BRCA2 | E731* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | PA | Case report |
| BRCA2 | E731* | Complete Match | Olaparib (PARP inhibitor) | Responsive | PRAD | Early trials |
| BRCA2 | E731* | Complete Match | Rucaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | E731* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | E731* | Complete Match | Veliparib;Cisplatin (PARP inhibitor;Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | E731* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | E731* | Complete Match | PARP inhibitor | Responsive | BRCA | Early trials |
| BRCA2 | E731* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | E731* | Different Alteration | PARP inhibitor | Responsive | OV | Pre-clinical |
| PDGFB | 3-UTRSNV | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | DFS | FDA guidelines |
| TP53 | R273C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (IntronicBlockSubstitution | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| TP53 | E68* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E68* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E68* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E68* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E68* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E68* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E68* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E68* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E68* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E68* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E68* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E68* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ESR1 | 3-UTRSNV), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| Variation | Gene | Transcript | Protein Alteration | Consequence | Tumour Driver | Oncogenic Classification |
|---|
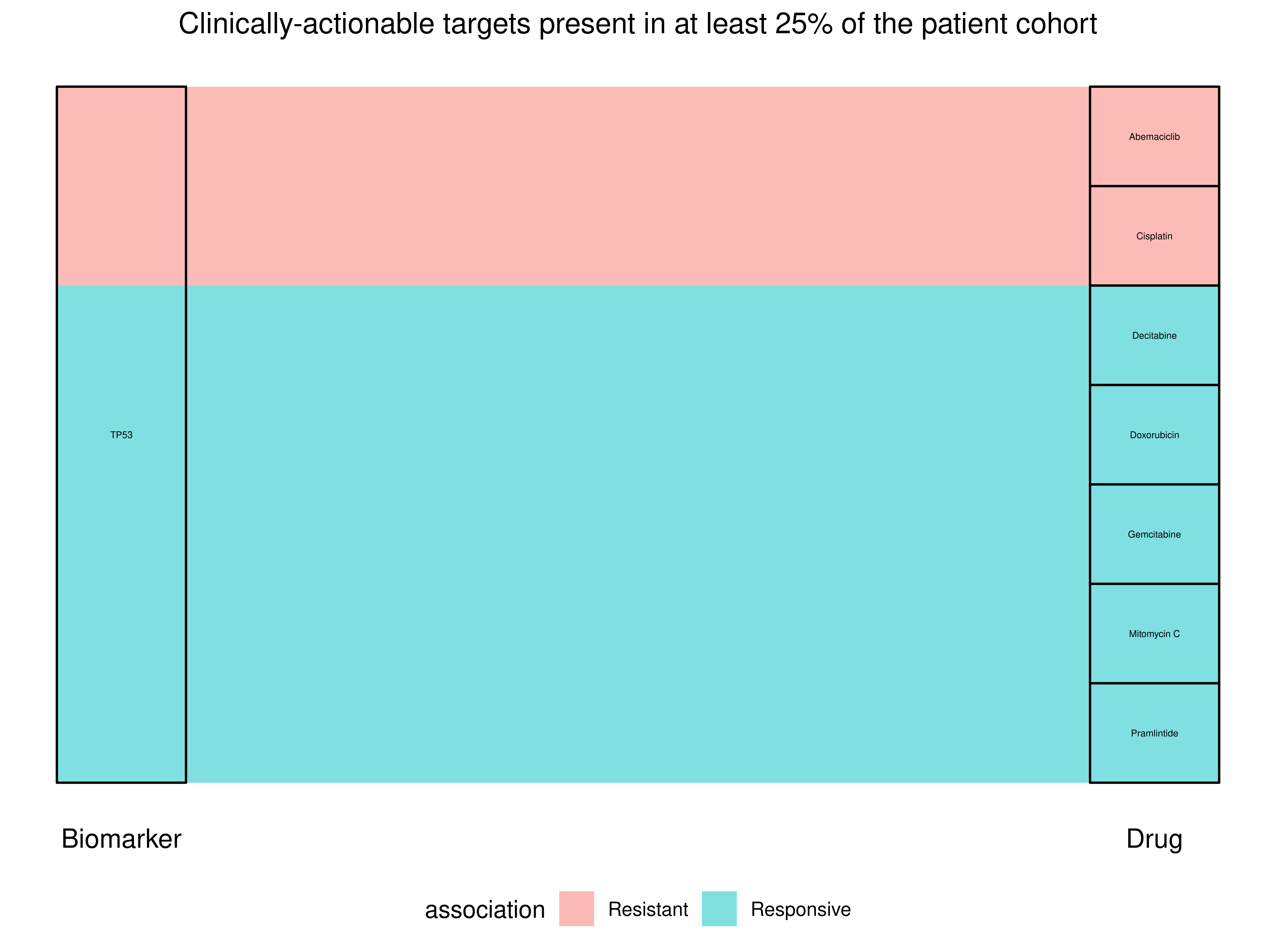
Druggable categories in the cohort
No meaningful categories found
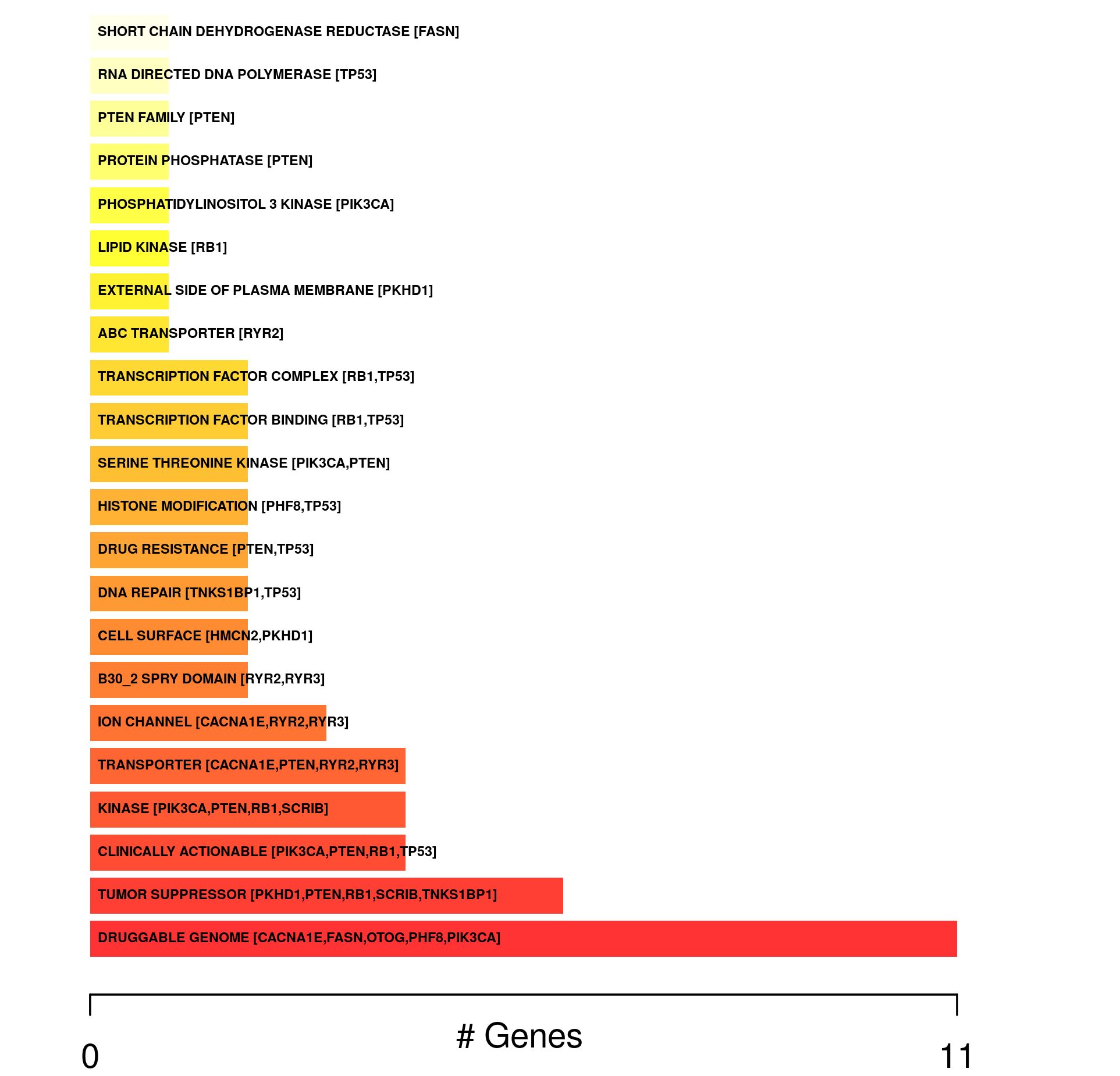
Focus the search to a gene of interest:
| Gene | Interaction Source | Interaction Types | Drug Claim Primary Name | Drug Name | ChEMBL ID |
|---|---|---|---|---|---|
| TP53 | CKB | CTX-1 | |||
| TP53 | CKB | Gemcitabine | GEMCITABINE | CHEMBL888 | |
| TP53 | ClearityFoundationClinicalTrial | GRANISETRON | GRANISETRON | CHEMBL289469 | |
| TP53 | CKB | epirubicin | EPIRUBICIN | CHEMBL417 | |
| TP53 | NCI | TOPICAL CORTICOSTEROIDS | |||
| TP53 | CKB | Trametinib | TRAMETINIB | CHEMBL2103875 | |
| TP53 | TALC | vaccine | AD.P53-DC | ||
| TP53 | CKB | Cisplatin | |||
| TP53 | NCI | YONDELIS | YONDELIS | CHEMBL297812 | |
| TP53 | CKB | Doxorubicin | DOXORUBICIN | CHEMBL53463 | |
| TP53 | CGI | MK-1775 | AZD-1775 | CHEMBL1976040 | |
| TP53 | CKB | Paclitaxel | PACLITAXEL | CHEMBL428647 | |
| TP53 | NCI | ZINC CHLORIDE | ZINC CHLORIDE | CHEMBL1200679 | |
| TP53 | CKB | PD-0325901 | PD-0325901 | CHEMBL507361 | |
| TP53 | CKB | PK11007 | |||
| TP53 | CKB | Temozolomide | TEMOZOLOMIDE | CHEMBL810 | |
| TP53 | CKB | TC-A2317 | |||
| TP53 | CKB | NU6027 | CHEMBL303958 | CHEMBL303958 | |
| TP53 | NCI | DAB | |||
| TP53 | CKB | IGF-1R antibody | |||
| TP53 | CKB | PT2399 | |||
| TP53 | CKB | Ifosfamide | IFOSFAMIDE | CHEMBL1024 | |
| TP53 | CIViC | SELUMETINIB (AZD6244) | SELUMETINIB | CHEMBL1614701 | |
| TP53 | CKB | EGFR antibody | |||
| TP53 | NCI | IL-12 | PROXYPHYLLINE | CHEMBL37390 | |
| TP53 | NCI | METHIMAZOLE | METHIMAZOLE | CHEMBL1515 | |
| TP53 | CKB | Cytarabine | CYTARABINE | CHEMBL803 | |
| TP53 | TTD | activator | CURAXIN CBLC102 | ||
| TP53 | CGI | Pramlintide | PRAMLINTIDE | CHEMBL2103758 | |
| TP53 | CIViC | CAPECITABINE | CAPECITABINE | CHEMBL1773 | |
| TP53 | CKB | Docetaxel | DOCETAXEL | CHEMBL92 | |
| TP53 | NCI | PROPYLTHIOURACIL | PROPYLTHIOURACIL | CHEMBL1518 | |
| TP53 | CKB | Ibrutinib | IBRUTINIB | CHEMBL1873475 | |
| TP53 | CKB | INC280 | CAPMATINIB | CHEMBL3188267 | |
| TP53 | CKB | Prodigiosin | PRODIGIOSIN | CHEMBL275787 | |
| TP53 | CKB | CGM097 | |||
| TP53 | CKB | SRA737 | |||
| TP53 | NCI | PUROMYCIN | PUROMYCIN | CHEMBL469912 | |
| TP53 | CKB | ABT-737 | CHEMBL376408 | CHEMBL376408 | |
| TP53 | CKB | Crizotinib | CRIZOTINIB | CHEMBL601719 | |
| TP53 | NCI | RAPAMYCIN | SIROLIMUS | CHEMBL413 | |
| TP53 | CKB | AZD6738 | AZD-6738 | CHEMBL3545178 | |
| TP53 | CKB | p53-SLP vaccine | |||
| TP53 | CKB | Irinotecan | IRINOTECAN | CHEMBL481 | |
| TP53 | NCI | TRIETHYLENETHIOPHOSPHORAMIDE | THIOTEPA | CHEMBL671 | |
| TP53 | NCI | GARLIC | GARLIC | CHEMBL2108463 | |
| TP53 | CKB | MK-8745 | |||
| TP53 | CIViC | AMGMDS3 | |||
| TP53 | CKB | Erlotinib | ERLOTINIB | CHEMBL553 | |
| TP53 | CKB | Pembrolizumab | PEMBROLIZUMAB | CHEMBL3137343 | |
| TP53 | NCI | VEGF | BEVACIZUMAB | CHEMBL1201583 | |
| TP53 | CKB | AMG 232 | |||
| TP53 | CKB | BEZ235 | DACTOLISIB | CHEMBL1879463 | |
| TP53 | CKB | Vemurafenib | VEMURAFENIB | CHEMBL1229517 | |
| TP53 | CKB | YW3-56 | |||
| TP53 | CKB | SAR405838 | |||
| TP53 | CGI | Cisplatin | |||
| TP53 | CGI | Abemaciclib | ABEMACICLIB | CHEMBL3301610 | |
| TP53 | CKB | PF-00477736 | PF-00477736 | CHEMBL3545137 | |
| TP53 | CKB | AMG 900 | AMG-900 | CHEMBL2140408 | |
| TP53 | CKB | GSK2830371 | |||
| TP53 | CKB | unspecified IGF-1R antibody | |||
| TP53 | NCI | DNA VACCINE | |||
| TP53 | CKB | Doxil | |||
| TP53 | CKB | AMG 337 | AMG-337 | CHEMBL3545212 | |
| TP53 | CKB | AZD6482 | AZD-6482 | CHEMBL2165191 | |
| TP53 | CGI | Decitabine | DECITABINE | CHEMBL1201129 | |
| TP53 | CIViC | NUTLIN-3A | |||
| TP53 | CKB | Bevacizumab | BEVACIZUMAB | CHEMBL1201583 | |
| TP53 | NCI | TRIFLUOPERAZINE | TRIFLUOPERAZINE | CHEMBL422 | |
| TP53 | CIViC | TAMOXIFEN | TAMOXIFEN | CHEMBL83 | |
| TP53 | CIViC | DOCETAXEL | DOCETAXEL | CHEMBL92 | |
| TP53 | CKB | ERBB3 antibody | |||
| TP53 | CKB | Tanespimycin | TANESPIMYCIN | CHEMBL109480 | |
| TP53 | CKB | JQ1 | |||
| TP53 | CKB | R547 | RG-547 | CHEMBL384304 | |
| TP53 | CKB | Selumetinib | SELUMETINIB | CHEMBL1614701 | |
| TP53 | CGI | AZD6738 | AZD-6738 | CHEMBL3545178 | |
| TP53 | CKB | Olaparib | OLAPARIB | CHEMBL521686 | |
| TP53 | NCI | TIRAPAZAMINE | TIRAPAZAMINE | CHEMBL50882 | |
| TP53 | CIViC | CISPLATIN | |||
| TP53 | CKB | Trifluridine | TRIFLURIDINE | CHEMBL1129 | |
| TP53 | CIViC | DOXORUBICIN | DOXORUBICIN | CHEMBL53463 | |
| TP53 | CKB | Epirubicin | EPIRUBICIN | CHEMBL417 | |
| TP53 | CKB | Radiotherapy | |||
| TP53 | CKB | NSC59984 | |||
| TP53 | CKB | Duvelisib | DUVELISIB | CHEMBL3039502 | |
| TP53 | CIViC | CETUXIMAB | CETUXIMAB | CHEMBL1201577 | |
| TP53 | NCI | MPA | MYCOPHENOLIC ACID | CHEMBL866 | |
| TP53 | CKB | Vorinostat | VORINOSTAT | CHEMBL98 | |
| TP53 | CKB | Alvespimycin | ALVESPIMYCIN | CHEMBL383824 | |
| TP53 | CKB | Daunoxome | |||
| TP53 | CKB | APR-246 | |||
| TP53 | CKB | Cyclophosphamide | CYCLOPHOSPHAMIDE | CHEMBL88 | |
| TP53 | CKB | APG-115 | |||
| TP53 | CKB | AZD2461 | |||
| TP53 | CKB | unspecified ERBB3 antibody | |||
| TP53 | CKB | MI-63 | |||
| TP53 | CIViC | CHEMOTHERAPY | |||
| TP53 | CKB | AZD5363 | AZD-5363 | CHEMBL2178577 | |
| TP53 | CKB | MLN0128 | INK-128 | CHEMBL3545056 | |
| TP53 | CKB | Nutlin-3a | |||
| TP53 | CKB | Dasatinib | DASATINIB | CHEMBL1421 | |
| TP53 | CKB | Carboplatin | CARBOPLATIN | CHEMBL1351 | |
| TP53 | NCI | CISPLATIN | |||
| TP53 | CKB | Sapanisertib | INK-128 | CHEMBL3545056 | |
| TP53 | CKB | NSC319726 | |||
| TP53 | CKB | p28 | |||
| TP53 | CKB | Dabrafenib | DABRAFENIB | CHEMBL2028663 | |
| TP53 | NCI | CAROTENOID | |||
| TP53 | CKB | DS-7423 | DS-7423 | CHEMBL3545248 | |
| TP53 | CKB | ONC201 | |||
| TP53 | CKB | Camptosar | |||
| TP53 | CKB | Ganetespib | GANETESPIB | CHEMBL2103879 | |
| TP53 | CKB | RO6839921 | |||
| TP53 | CKB | LY3009120 | LY-3009120 | CHEMBL3545195 | |
| TP53 | CKB | Ad.p53-DC vaccine | |||
| TP53 | CKB | Flourouracil | |||
| TP53 | NCI | RITUXIMAB | RITUXIMAB | CHEMBL1201576 | |
| TP53 | NCI | SAPONIN | |||
| TP53 | NCI | MCP-1 | |||
| TP53 | NCI | DES | DIETHYLSTILBESTROL | CHEMBL411 | |
| TP53 | NCI | R-FLURBIPROFEN | TARENFLURBIL | CHEMBL190083 | |
| TP53 | NCI | HALOPERIDOL | HALOPERIDOL | CHEMBL54 | |
| TP53 | CKB | PF-04217903 | PF-04217903 | CHEMBL2001019 | |
| TP53 | CKB | Oxaliplatin | OXALIPLATIN | CHEMBL414804 | |
| TP53 | CKB | Serdemetan | |||
| TP53 | TALC | inhibitor | BORTEZOMIB | BORTEZOMIB | CHEMBL325041 |
| TP53 | CKB | CPUY201112 | |||
| TP53 | CKB | MVAp53 | |||
| TP53 | CKB | Pazopanib | PAZOPANIB | CHEMBL477772 | |
| TP53 | CGI | Mitomycin C | MITOMYCIN | CHEMBL105 | |
| TP53 | NCI | FAS LIGAND | RIZATRIPTAN | CHEMBL905 | |
| TP53 | CKB | thioureidobutyronitrile | |||
| TP53 | CKB | Alpelisib | ALPELISIB | CHEMBL2396661 | |
| TP53 | CKB | Nutlin-3 | NUTLIN-3 | CHEMBL191334 | |
| TP53 | CKB | Abemaciclib | ABEMACICLIB | CHEMBL3301610 | |
| TP53 | CKB | Navitoclax | NAVITOCLAX | CHEMBL443684 | |
| TP53 | CKB | PF-06463922 | LORLATINIB | CHEMBL3286830 | |
| TP53 | CIViC | ADJUVANT CHEMOTHERAPY | |||
| TP53 | CKB | ALRN-6924 | |||
| TP53 | CKB | GDC-0425 | RG-7602 | CHEMBL3545007 | |
| TP53 | CKB | SP600125 | SP-600125 | CHEMBL1725279 | |
| TP53 | CKB | MPI-0479605 | HEMICHOLINIUM-3 | CHEMBL268697 | |
| TP53 | CKB | Avastin | BEVACIZUMAB | CHEMBL1201583 | |
| TP53 | NCI | VESNARINONE | VESNARINONE | CHEMBL17423 | |
| TP53 | CKB | EMD 1214063 | Tepotinib | CHEMBL3402762 | |
| TP53 | CKB | Pimasertib | PIMASERTIB | CHEMBL2107832 | |
| TP53 | CKB | RG7112 | |||
| TP53 | CKB | LB-100 | |||
| TP53 | CKB | ||||
| TP53 | CKB | AZD7762 | AZD-7762 | CHEMBL2041933 | |
| TP53 | CKB | HDM201 | |||
| TP53 | NCI | L-744,832 | CHEMBL1221512 | CHEMBL1221512 | |
| TP53 | CKB | PF-05212384 | GEDATOLISIB | CHEMBL592445 | |
| TP53 | CKB | Etoposide | ETOPOSIDE | CHEMBL44657 | |
| TP53 | CKB | ReACp53 | |||
| TP53 | NCI | ENALAPRIL | ENALAPRIL | CHEMBL578 | |
| TP53 | NCI | SELECTIVE ESTROGEN RECEPTOR MODULATORS | |||
| TP53 | CKB | unspecified EGFR antibody | |||
| TP53 | CKB | MK-1775 | AZD-1775 | CHEMBL1976040 | |
| TP53 | CGI | Doxorubicin | DOXORUBICIN | CHEMBL53463 | |
| TP53 | CKB | LY411575 | CHEMBL392068 | CHEMBL392068 | |
| TP53 | CKB | PI-3065 | |||
| TP53 | CGI | Gemcitabine | GEMCITABINE | CHEMBL888 | |
| TP53 | NCI | METHYLPREDNISOLONE | METHYLPREDNISOLONE | CHEMBL650 | |
| TP53 | CIViC | CARBOPLATIN | CARBOPLATIN | CHEMBL1351 | |
| TP53 | CKB | Demcizumab | DEMCIZUMAB | CHEMBL2109384 | |
| TP53 | CIViC | RG7112 | |||
| TP53 | CKB | Sirolimus | SIROLIMUS | CHEMBL413 | |
| TP53 | NCI | INTERFERON ALPHA-2B | INTERFERON ALFA-2B | CHEMBL1201558 | |
| TP53 | CKB | Daunorubicin | DAUNORUBICIN | CHEMBL178 | |
| TP53 | NCI | GOSSYPOL | GOSSYPOL | CHEMBL51483 | |
| TP53 | CKB | BLZ945 | |||
| TP53 | CKB | PHA-680632 | PHA-680632 | CHEMBL363160 | |
| TP53 | CKB | Topotecan | TOPOTECAN | CHEMBL84 | |
| TP53 | CKB | 7RH | |||
| TP53 | CKB | LGX818 | ENCORAFENIB | CHEMBL3301612 | |
| TP53 | NCI | INOSITOL | INOSITOL | CHEMBL1222251 | |
| TP53 | TALC | vaccine | EP-2101 | ||
| TP53 | CIViC | OXALIPLATIN | OXALIPLATIN | CHEMBL414804 | |
| TP53 | CKB | ABT-263 | NAVITOCLAX | CHEMBL443684 | |
| TP53 | CKB | MK-8242 | |||
| TP53 | CKB | VX-970 | VX-970 | CHEMBL3545202 | |
| TP53 | CKB | CP-31398 | |||
| TP53 | NCI | URSODEOXYCHOLIC ACID | URSODIOL | CHEMBL1551 | |
| TP53 | CKB | Ad5CMV-p53 gene | |||
| TP53 | NCI | PACLITAXEL | PACLITAXEL | CHEMBL428647 | |
| TP53 | CKB | CEP-8983 | |||
| TP53 | CKB | Panitumumab | PANITUMUMAB | CHEMBL1201827 | |
| TP53 | NCI | STREPTONIGRIN | STREPTONIGRIN | CHEMBL11417 | |
| TP53 | CKB | Seliciclib | SELICICLIB | CHEMBL14762 | |
| TP53 | CKB | PD0166285 |
There are patients available from the BCN Biobank that match the selected/a subset of the criteria. Explore the data available for these patients using our Cohort Browser.
Researchers can request samples from BCNB website to support their research activities.
*Based on your filtering criteria you can view the number of available patients and associated samples in BCNBIn order to apply for samples, please use the BCNB Request System to submit an Expression of interest (EOI) form.
If you require further details please contact us at tissue.bank@breastcancernow.org
| Patient ID | Age at Diagnosis | Sex | Ethnicity | Menopausal Status | Family History | Family History Relative | BRCA Status | Definition | ER | PR | HER2 | Tumour Grade | Tumour Size | Lymph Node | Survival Status | Followup (Years) | Recurrence | Metastasis | Genetic Ancestry | Location | hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1007 | 77 | female | Not specified | postmenopausal | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 20 | Negative | Alive | 11.3 | NA | NA | European | Barts | LP3001415-DNA_H09 |
| 1203 | 43 | female | White - British | premenopausal | yes | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 27 | Negative | Alive | 8.7 | NA | NA | European | Barts | LP3001381-DNA_F01 |
| 1263 | 80 | female | White - British | postmenopausal | yes | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 38 | Negative | Dead | 7.3 | NA | NA | European | Barts | LP3001415-DNA_F09 |
| 1506 | 46 | female | White - British | postmenopausal | yes | yes | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 35 | Negative | Alive | 9.2 | NA | NA | European | Barts | LP3001381-DNA_C01 |
| 1509 | 62 | female | Black or Black British - Caribbean | postmenopausal | yes | yes | unknown | primary | Borderline | Negative | Negative | Grade 3 | 45 | Positive | Alive | 7.8 | NA | CL | African | Barts | LP3001415-DNA_D08 |
| 1633 | 46 | female | White - Any other background | premenopausal | yes | no | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 22 | Negative | Alive | 7.8 | NA | NA | European | Barts | LP3001415-DNA_G10 |
| 1666 | 66 | female | White - British | postmenopausal | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 13 | Negative | Alive | 8.5 | NA | NA | European | Barts | LP3001415-DNA_C09 |
| 1759 | 62 | female | White - Any other background | postmenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 18 | Positive | Alive | 6.6 | NA | NA | European | Barts | LP3001415-DNA_C08 |
| 1903 | 76 | female | White - British | postmenopausal | unknown | unknown | unknown | primary | Negative | Negative | Negative | Grade 3 | 28 | Negative | Alive | 8.2 | NA | NA | European | Barts | LP3001381-DNA_A01 |
| 1908 | 42 | female | White - Any other background | premenopausal | yes | no | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 23 | Positive | Alive | 8.3 | NA | NA | European | Barts | LP3001415-DNA_H08 |
| 1941 | 74 | female | White - British | postmenopausal | yes | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 15 | Negative | Dead | 6.8 | NA | NA | European | Barts | LP3001415-DNA_B10 |
| 1979 | 55 | female | Black or Black British - Caribbean | not recorded | yes | yes | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 6 | Negative | Alive | 7.8 | NA | NA | African | Barts | LP3001415-DNA_C10 |
| 1987 | 70 | female | White - Irish | postmenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 22 + 20 + 4 | Positive | Dead | 5.5 | yes | yes | European | Barts | LP3001415-DNA_C04 |
| 2061 | 58 | female | White - British | postmenopausal | unknown | unknown | unknown | primary | Borderline | Negative | Negative | Grade 3 | 24 | Negative | Alive | 7.8 | NA | CL | European | Barts | LP3001415-DNA_B08 |
| 2163 | 39 | female | White - Any other background | premenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 60 | Positive | Alive | 7.3 | NA | NA | European | Barts | LP3001381-DNA_A02 |
| 2527 | 82 | female | Black or Black British - Any other Black background | postmenopausal | unknown | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 20 | Negative | Dead | 4.3 | yes | yes | African | Barts | LP3001381-DNA_B02 |
| 2536 | 66 | female | Black or Black British - Caribbean | postmenopausal | yes | no | unknown | primary | Negative | Negative | Negative | Grade 2 | 16 + 7 | Negative | Alive | 4.5 | NA | NA | African | Barts | LP3001415-DNA_A07 |
| 2609 | 59 | female | White | postmenopausal | yes | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 58 | Negative | Alive | 5.9 | NA | NA | Admix | Barts | LP3001415-DNA_D07 |
| 2680 | 50 | female | White - Any other background | perimenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 19 | Negative | Alive | 3.9 | NA | NA | American | Barts | LP3001415-DNA_B09 |
| 2753 | 74 | female | Black or Black British - Caribbean | postmenopausal | unknown | unknown | unknown | primary | Negative | Negative | Negative | Grade 3 | 42 | Positive | Alive | 5.3 | NA | NA | African | Barts | LP3001415-DNA_A08 |
| 2819 | 44 | female | Asian or Asian British - Indian | premenopausal | no | no | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 22 | Negative | Alive | 4.3 | NA | NA | South Asian | Barts | LP3001381-DNA_E01 |
| 2842 | 51 | female | Asian or Asian British - Bangladeshi | premenopausal | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 13 | Negative | Alive | 9.8 | NA | CL | South Asian | Barts | LP3001415-DNA_A09 |
| 2953 | 66 | female | White - British | postmenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 17 | Negative | Alive | 4.7 | NA | NA | European | Barts | LP3001415-DNA_A10 |
| D10271 | 75 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 20 | Negative | Alive | 3.3 | NA | NA | European | Dundee | LP3001415-DNA_G02 |
| D10466 | 75 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 39 | Negative | Dead | 2.6 | NA | NA | European | Dundee | LP3001415-DNA_G03 |
| D10540 | 64 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 17 | Negative | Dead | 2.7 | NA | NA | European | Dundee | LP3001415-DNA_G04 |
| D10921 | 49 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 18 | Negative | Alive | 0.4 | NA | NA | European | Dundee | LP3001415-DNA_C06 |
| D11098 | 57 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 10 | Negative | Alive | 2.2 | NA | NA | European | Dundee | LP3001415-DNA_A01 |
| D11120 | 70 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 40 | Positive | Dead | 0.5 | NA | NA | European | Dundee | LP3001415-DNA_H02 |
| D11278 | 71 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 17 | Negative | Alive | 1.0 | NA | NA | European | Dundee | LP3001415-DNA_H03 |
| D11327 | 62 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 32 | Negative | Alive | 0.5 | NA | NA | European | Dundee | LP3001415-DNA_H04 |
| D11718 | 83 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | NA | 73 | Negative | NA | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_H05 |
| D11719 | 49 | female | Not specified | premenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 14 | Negative | Alive | 1.1 | NA | NA | European | Dundee | LP3001415-DNA_D01 |
| D11766 | 77 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 85 | Negative | Dead | 0.3 | NA | NA | European | Dundee | LP3001415-DNA_D02 |
| D12258 | 46 | female | Not specified | premenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 15 | Negative | Alive | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_D03 |
| D12419 | 53 | female | Not specified | perimenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 2 | 25 | Positive | Alive | 1.2 | NA | NA | European | Dundee | LP3001415-DNA_D04 |
| D12507 | 73 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 21 | Negative | Alive | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_G05 |
| D12646 | 36 | female | Not specified | premenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 22 | Negative | Alive | 0.5 | NA | NA | European | Dundee | LP3001415-DNA_D06 |
| D12695 | 52 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 2 | 2 | Negative | Alive | 2.1 | NA | NA | European | Dundee | LP3001415-DNA_E01 |
| D12775 | 53 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 29 | Negative | Alive | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_E02 |
| D12815 | 62 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 100 | Positive | Alive | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_E04 |
| D12883 | 46 | female | Not specified | premenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 1 | 11 | NA | Alive | 0.8 | NA | NA | European | Dundee | LP3001415-DNA_E05 |
| D12903 | 64 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 45 | Positive | Alive | 0.8 | NA | NA | European | Dundee | LP3001415-DNA_E06 |
| D13118 | 54 | female | Not specified | perimenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 39 | Positive | Alive | 0.6 | NA | NA | European | Dundee | LP3001415-DNA_F02 |
| D13137 | 56 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 19 | Negative | Alive | 0.4 | NA | NA | European | Dundee | LP3001415-DNA_F03 |
| D13337 | 66 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 33 | Negative | NA | 0.2 | NA | NA | European | Dundee | LP3001415-DNA_F04 |
| D13350 | 68 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 2 | 8 | Negative | Alive | 0.4 | NA | NA | European | Dundee | LP3001415-DNA_B01 |
| D13432 | 72 | female | Not specified | not recorded | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 0 | Positive | Alive | 0.8 | NA | NA | European | Dundee | LP3001415-DNA_F05 |
| D13526 | 66 | female | Not specified | postmenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 20 | Negative | Alive | 0.7 | NA | NA | European | Dundee | LP3001415-DNA_F06 |
| D9892 | 50 | female | Not specified | premenopausal | unknown | unknown | unknown | primary | NA | NA | Negative | Grade 3 | 23 | Positive | Alive | 3.7 | NA | NA | European | Dundee | LP3001415-DNA_G06 |
| LS12-098 | 45 | female | White - British | premenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 2 | 9 | Negative | Alive | 5.6 | NA | NA | European | Leeds | LP3001415-DNA_C02 |
| LS12-122 | 78 | female | White - British | postmenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 17 | Negative | Alive | 5.5 | NA | NA | European | Leeds | LP3001415-DNA_G07 |
| LS12-128 | 41 | female | White - British | premenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 16 | Negative | Alive | 5.3 | NA | NA | European | Leeds | LP3001415-DNA_F01 |
| LS12-297 | 78 | female | White - British | postmenopausal | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 45 | Negative | Dead | 3.5 | yes | yes | European | Leeds | LP3001415-DNA_H01 |
| LS12-379 | 68 | female | White - British | postmenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 40 | Negative | Alive | 5.3 | yes | yes | European | Leeds | LP3001415-DNA_A02 |
| LS12-417 | 64 | female | White - British | postmenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 2 | 1.5 | NA | Alive | 4.4 | NA | NA | European | Leeds | LP3001415-DNA_B11 |
| LS13-03209 | 54 | female | White - British | premenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 60 | Positive | Dead | 1.3 | yes | yes | European | Leeds | LP3001415-DNA_A04 |
| LS13-03399 | 61 | female | White - British | postmenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 19 | Negative | Alive | 4.1 | NA | NA | European | Leeds | LP3001415-DNA_B02 |
| LS13-03477 | 69 | female | White - Any other background | not recorded | none recorded | none recorded | unknown | primary | NA | NA | NA | Grade 3 | NS | Negative | Alive | NA | NA | NA | European | Leeds | LP3001415-DNA_A05 |
| LS14-02842 | 69 | female | White - British | not recorded | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 130 | Positive | Dead | 0.25 | NA | yes | European | Leeds | LP3001415-DNA_B06 |
| LS15-02515 | 48 | female | White - British | perimenopausal | unknown | unknown | unknown | primary | Negative | Negative | Negative | Grade 3 | 19 | Negative | Alive | 2.33 | NA | NA | European | Leeds | LP3001415-DNA_A03 |
| LS15-02568 | 62 | female | White - British | postmenopausal | yes | no | unknown | recurrence | Negative | Negative | Negative | Grade 3 | 9 | Negative | Alive | 3.0 | NA | NA | European | Leeds | LP3001415-DNA_G01 |
| LS16-4577 | 51 | female | White - British | perimenopausal | none recorded | none recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 27 | Positive | Alive | 1.08 | NA | NA | European | Leeds | LP3001415-DNA_B03 |
| LS16-4682 | 60 | female | White - British | postmenopausal | yes | yes | unknown | primary | Negative | Negative | Negative | Grade 3 | 24 | Positive | Alive | 0.75 | NA | NA | European | Leeds | LP3001415-DNA_B04 |
| LS17-4826 | 42 | female | White - British | not recorded | yes | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 42 | Negative | Alive | 0.67 | NA | NA | European | Leeds | LP3001415-DNA_B05 |
| N12/001605 | 59 | female | White - British | unknown | unknown | unknown | NA | primary | Negative | Negative | Negative | Grade 3 | 13 | Negative | Alive | 6.42 | NA | NA | European | Nottingham | LP3001415-DNA_G09 |
| N12/001734 | 37 | female | White - British | unknown | not recorded | not recorded | unknown | primary | Positive | Negative | Negative | Grade 3 | 20 | Negative | Alive | 5.0 | NA | NA | European | Nottingham | LP3001415-DNA_D10 |
| N12/003761 | 50 | female | White - British | postmenopausal | not recorded | not recorded | unknown | primary | Negative | Negative | Negative | Grade 3 | 25 | Negative | Alive | 5.67 | NA | NA | European | Nottingham | LP3001415-DNA_H06 |
| N13/000457 | 61 | female | White - British | unknown | no | no | unknown | primary | Positive | Negative | Negative | Grade 2 | 13 | Negative | Alive | 6.33 | NA | CL | European | Nottingham | LP3001381-DNA_D01 |
| N13/000560 | 49 | female | White - British | postmenopausal | yes | yes | negative | contralateral | Negative | Negative | Negative | Grade 3 | 16 | Positive | Alive | 6.0 | NA | yes | European | Nottingham | LP3001415-DNA_E09 |
| N13/005968 | 45 | female | White - British | premenopausal | yes | yes | BRCA1 | primary | Negative | Negative | Negative | Grade 3 | 26 | Negative | Alive | 5.58 | NA | NA | European | Nottingham | LP3001415-DNA_A06 |
| N14/001416 | 49 | female | White - British | postmenopausal | unknown | unknown | unknown | contralateral | Negative | Negative | Negative | Grade 3 | 24 | Negative | Dead | 4.0 | NA | CL | European | Nottingham | LP3001415-DNA_E10 |
| N14/001950 | 71 | female | White - British | unknown | unknown | unknown | unknown | primary | Negative | Negative | Negative | Grade 3 | 31 | Negative | Alive | 3.25 | NA | NA | European | Nottingham | LP3001415-DNA_E08 |
| N14/002100 | 54 | female | White - British | unknown | unknown | unknown | unknown | primary | Negative | Negative | Negative | Grade 3 | 50 | Negative | Dead | 4.08 | NA | NA | European | Nottingham | LP3001415-DNA_D09 |
| N16/000331 | 50 | female | White - British | postmenopausal | no | no | unknown | primary | Negative | Negative | Negative | Grade 3 | 18 | Negative | Dead | 3.58 | yes | yes | European | Nottingham | LP3001415-DNA_H10 |
| N17/008421 | 51 | female | White - British | unknown | yes | yes | negative | primary | Negative | Negative | Negative | Grade 3 | 27 | Positive | Alive | 2.17 | NA | NA | European | Nottingham | LP3001415-DNA_C07 |